Five Design Principles for creating equitable learning experiences
|
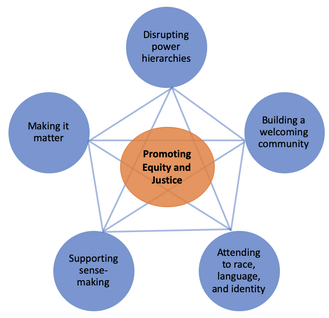
The five design principles, emerging from research on
when and under which conditions students from non-dominant communities engage in science meaningfully, guide our design and enactment of curricula. They are: (a) making it matter, (b) supporting sense-making, (c) attending to race, language, and identities, (d) building a welcoming community, and (e) disrupting power hierarchies (see the details in Kang & Nation, under review). |
How can we choose the best material for a reusable item?
Trends and Reactivity Unit
|
Unit: Trends and Reactivity
Grade Level(s): 10-12 Duration: 5 weeks The major task of this unit is to design a unique piece of 'keepsake' that lasts a long time and can be passed to the next generations of their families. The unit begins by sharing stories about a unique family keepsake, inviting students to think why some items last and why some others doesn't last. The students learn to explain how & why materials break down/corrode over time due to reactions with their environment (water & oxygen in particular). |
How can we design a chemically-powered heating or cooling device for people who don't have access to electricity?
Thermodynamics and Stoichometry Unit
|
Unit: Thermodynamics and Stoichometry
Grade Level(s): 10-12 Duration: 4 weeks In this unit, students are introduced to the idea that when a chemical reaction occurs, there is some movement of heat (energy). They are introduced to the terminologies, endothermic & exothermic reaction to describe the movement of heat (energy) transfer in and out of a system. In this unit, we are leveraging the idea of heat/energy transfer. Students are tasked to design a chemically powered heating or cooling device for people who do not have electricity. In the processes of designing this device, students are tasked to figure out which material they should choose to heat up or cool down, how much, and why. |
What are some of the causes and effects of wildfires?
The Rate of chemical Reactions and Equilibrium
|
Unit: The rate of chemical reaction and equilibrium
Grade Level(s): 9-12 high school Duration of the unit: about 5 weeks This unit begins with students relating their personal experiences with local wildfires--especially the Canyon 2 Fire that resulted in a cancelled school day in the 2017-18 school year. They compare the way Tustin looks today to how it looked about 100 years ago when Tustin High School opened (1921) and propose an initial model for why wildfires are worse now than they were 100 years ago in relation to the change of carbon dioxide in the atmosphere. Over the course of the unit, students are guided to expand/ revise their ideas by engaging in various activities. Specifically, they learn about systems thinking and the chemistry concepts of rates and equilibrium. Students explored how temperature and concentration affect the rate of a chemical reaction (the rate of wildfire outbreak and spread) via a small “rocket” launch activity. They use the carbon cycle to explain the changing equilibrium and predict the results of stresses added to that system. Students investigate climate change by determining their own carbon footprint as well as considering climate change denier claims and using evidence from data tables, graphs, and charts to disprove them. Throughout the unit, students revised their initial explanatory model a few times as they gather new ideas and evidence. Students are also introduced indigenous people’s sense-making about the recent wildfire in CA--how they make sense of it and how they have been harmoniously living and managing the ecosystem as a part of the nature using “controlled burn.” Students construct a final model and then propose a solution to lessen the severity of wildfires. Read more about this unit here. |
image source: https://abcnews.go.com/US/wireStory/remote-towns-evacuated-california-wildfire-grows-72863443